Introduction
Type 2 diabetes is associated with an increased risk of vascular complications, in particular macroangiopathy, which is the leading cause of mortality following coronary ischemic disease, stroke or other pathological conditions, including heart failure (1-3). As reported recently by Fitchett et al., heart failure has now emerged as a severe complication in diabetic subjects due to atherosclerotic and/or microvascular myocardial changes (4). Several reports confirmed that survival was lower in diabetic patients in the presence of heart failure: 40% of individuals diagnosed with both diabetes and heart insufficiency will die within the three years vs. only 4% of those diagnosed with diabetes alone (4). Conversely, patients with chronic heart failure who developed type 2 diabetes had also a poorer prognosis in terms of overall mortality, as reported by MacDonald et al. (5).
In this context, antihyperglycemic agents with a collateral “cardio protective” effect in terms of major adverse cardiovascular (CV) events (MACE) and/or heart failure are therefore potentially of main clinical interest in the treatment of type 2 diabetes, as also recently mentioned in the ADA-EASD recommendations (6,7).
SGLT-2 inhibitors are glucoretic agents characterized by a high antihyperglycemic efficacy in the setting of normal renal function (8,9). Moreover, as demonstrated in the EMPA-REG OUTCOME (10) and CANVAS (11) studies, in type 2 diabetic patients who had (in majority) established CV disease, they are associated with a significant reduction in CV events, in particular in the hospitalization rate for heart failure (approximatively -40% vs. placebo) (10,11). Observational data, especially in the CVD- Real study, are in phase with the results of these two important randomized trials published in the New England Journal of Medicine (10-13).
Dapagliflozin (Forxiga®) is a selective inhibitor of glucose cotransporter 2 that blocks glucose resorption in the proximal tubule of the kidney, promoting thereby glycosuria, weight loss and reduction in blood pressure levels (9).
The aim of this paper is to propose a state of the art concerning dapagliflozin in the context of the recently published DECLARE-TIMI 58 Trial (for Dapagliflozin in cardiovascular Events-Thrombolysis In Myocardial Infarction 58) (14).
DECLARE STUDY
Patients and methods
Subjects with type 2 diabetes, aged 40 years or older, who had or were at risk for atherosclerotic CV disease, were randomly assigned to receive, in addition to their usual antihyperglycemic therapy (which was at the discretion of their treating physician), either dapagliflozin (10mg/day) or a matching placebo. Eligible subjects had an HbA1c level between 6.5 and 12.0% as well as a creatinine clearance of 60 ml or more per minute.
Primary efficacy outcomes were (a) MACE, defined as CV death, myocardial infarction or ischemic stroke and (b) a composite of cardiovascular death or hospitalization for heart failure. Secondary prespecified efficacy outcomes were (a) a renal composite (> 40% decrease in estimated glomerular filtration rate to < 60 ml/minute per 1.73 m2, new end-stage renal disease or death from renal or cardiovascular causes), and (b) death from any cause. The follow-up of patients during trial was every six months.
Synopsis of main results
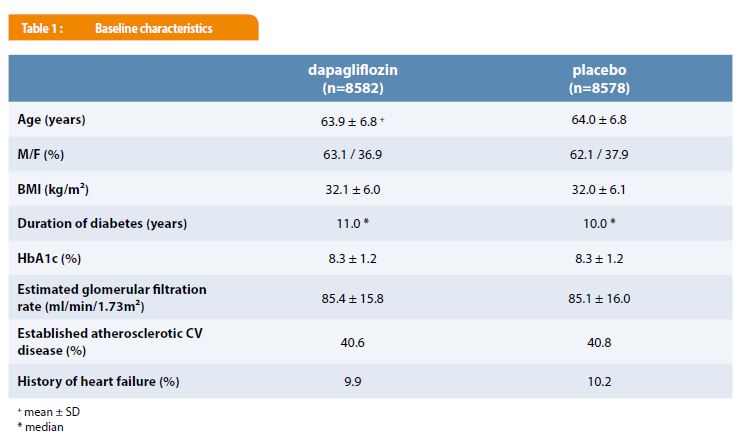
A total of 17 160 participants were included in the trial with 6 974 subjects (40.6%) with established atherosclerotic CV disease and 10 186 (59.4%), with multiple CV risk factors. They were followed for a median time of 4.2 years. As shown in Table 1, there were no significant differences in the baseline clinical and biological characteristics between the dapagliflozin and the placebo groups. Glucose-lowering and CV therapies were also comparable in the subjects receiving dapagliflozin or placebo. During the trial, treatment with dapagliflozin was associated with a weight loss of 1.8 kg and a reduction of systolic and diastolic blood pressure respectively of 2.7 and 0.7 mmHg vs. placebo. HbA1c decreased from 8.3 to 7.9% and from 8.3 to 8.1% in the dapagliflozin and placebo groups respectively.
The primary CV outcomes results are summarized in Table 2. Thus, in a first statistical step, dapagliflozin met the prespecified criterion for non-inferiority for MACE vs. placebo in the primary safety outcome analysis (p < 0.001). In a second step, in terms of primary efficacy outcomes, it did not result in a significant lower rate of MACE vs. placebo (8.8 vs. 9.4%, p = 0.17) (NS) (Table 2). In contrast, dapagliflozin was associated with a significant reduction in the rate of CV death or hospitalization for heart failure (4.9 vs. 5.8%, p = 0.005), as shown in Table 2. The difference in the latter composite outcome was mainly the consequence of a lower rate of hospitalization for heart failure in the dapagliflozin group (HR = 0.73 [95% CI : 0.61-0.88]), while there was no significant difference in the rate of CV death (HR = 0.98% [CI: 0.82-1.17]). It is essential to mention that the efficiency of dapagliflozin was observed in both subgroups of individuals with established atherosclerotic CV disease and in those with “only” CV risk factors (Table 2). Interestingly, the hazard ratio for this primary efficacy outcome was slightly lower in patients with an eGFR < 60 vs. > 90 ml/min/1.73m2 (HR: 0.78 [CI: 0.55-1.09] vs.0.96 [CI 0.77-1.19], p = 0.37 for interaction). The rate of death from any cause was comparable in the two groups (6.2 vs. 6.6% in the dapagliflozin and placebo groups, respectively). A renal event occurred in 4.3% in the dapagliflozin group and in 5.6% in the placebo group (HR: 0.76; [0.67-0.87]). Fewer patients in the dapagliflozin group (34.1%) vs. the placebo (36.2%) reported a severe adverse event (p < 0.001). As in previous SGLT-2 inhibitors studies, genital infections were more frequent in subjects receiving dapagliflozin vs. placebo (0.9 vs. 0.1%, p <0.001). Diabetic ketoacidosis was observed only in 0.3% of the subjects treated with dapagliflozin. The rate of amputations and fractures was similar in the two groups.

Discussion and conclusions
DECLARE TIMI 58 is a large trial assessing cardiovascular and renal outcomes with the SGLT-2 inhibitor dapagliflozin (14). Patients with type 2 diabetes and established CV disease or at high risk for CV events who were treated with dapagliflozin had a lower rate in the primary outcome of CV death or hospitalization for heart failure than subjects receiving placebo. The latter finding mainly reflected a lower rate of hospitalization for heart failure. These results are in phase with previous SGLT-2 inhibitors trials (EMPA-REG OUTCOME and CANVAS) demonstrating clearly favorable CV effects in patients with type 2 diabetes, including a statistical reduction in the risk of hospitalization for heart failure (10,11,15,16). The DECLARE-TIMI 58 trial however adds substantially to the literature since the study evidenced a statistical significant benefit in terms of a primary outcome (including CV death or hospitalization for heart failure) in a broader type 2 diabetic population with approximately 60% of subjects in primary CV prevention (without a past history of CV events). These results also extend previous interesting data in the field from the observational CVD-Real trial (12,13).
The present study did not find that dapagliflozin resulted in a lower rate of MACE or CV/overall deaths vs. placebo, a finding contrasting with data from previous trials, in particular EMPA-REG OUTCOME (10,11). This could be due to relevant differences in the clinical profiles of diabetic patients included in these studies. As already mentioned, in the DECLARE-TIMI 58, there was a high proportion of patients in primary CV prevention (vs. < 1% in EMPA-REG OUTCOME trial). Moreover, basal characteristics in terms of renal status were also somewhat different as shown in Table 3: the DECLARE-TIMI 58 study included only 7% of patients with an eGFR lower than 60 ml/min/1.73 m2 vs. 25% in the EMPA-REG OUTCOME (or 20% in CANVAS) (11,17).

EASD and ADA in 2018 indicate that, in type 2 diabetic patients with a past history of CV disease, heart failure or chronic nephropathy, SGLT-2 inhibitors and GLP-1 agonists are recommended in a dual therapy approach after metformin failure (6,7). In a very recent metanalysis, including more than 34 000 patients (60.21% with established atherosclerotic CV disease), Zelniker et al. demonstrated that SGLT-2 inhibitors had globally moderate benefits in MACE that were indeed confined to patients with a past history of atherosclerotic CV disease (HR: 0.89 [CI: 0.83-0.96], p =0.014), but major benefits in reducing hospitalization for heart failure (HR: 0.77 [CI 0.71-0.84], p < 0.0001) and progression of renal disease (HR: 0.55 [CI 0.48-0.64], p<0.01), irrespective of whether or not they had a macroangiopathy, chronic kidney disease or heart failure (18). In view of these data, Verma et al. even suggested that SGLT-2 inhibitors should be considered as first line therapy after metformin in most people with type 2 diabetes, regardless of an existing atherosclerotic CV disease or a history of heart failure (19).
As far as side effects of gliflozins are concerned, the rate of severe genital infections and diabetic ketoacidosis, even if higher, as expected, in the dapagliflozin group remained very low throughout the study in all the patients. It is also of interest to mention that the rate of amputation, fractures or volume depletion was comparable in the two groups of patients.
In conclusion, dapagliflozin administration resulted, in a broad population (primary and secondary prevention), in a significant lower rate of cardiovascular deaths and hospitalizations for heart failure vs. placebo, reflecting mainly a lower rate of hospitalizations for heart insufficiency. A reduction in terms of renal outcomes was also reported. Together, these observations are of main clinical relevance in the context of the high prevalence and severity of heart failure and nephropathy in type 2 diabetes. Clinicians should be aware of this new progress in the treatment of patients with type 2 diabetes with and without a medical history of macroangiopathy or heart failure.
Correspondance
Cliniques universitaires Saint-Luc
Service d’Endocrinologie et Nutrition
Avenue Hippocrate 10
B-1200 Bruxelles, Belgique
E-mail: martin.buysschaert@uclouvain.be
Références
- Buysschaert M. Diabétologie Clinique, 4e Edition, De Boeck, Louvain-la-Neuve, Paris, 2011.
- Hansen MB, Jensen ML, Carstensen B. Causes of death among diabetic patients in Denmark. Diabetologia. 2012 Feb;55(2):294-302.
ouvrir dans Pubmed - Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al.; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011 Mar 3;364(9):829-841.
ouvrir dans Pubmed - Fitchett DH, Udell JA, Inzucchi SE. Heart failure outcomes in clinical trials of glucose-lowering agents in patients with diabetes. Eur J Heart Fail. 2017 Jan;19(1):43-53.
ouvrir dans Pubmed - MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al.; CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008 Jun;29(11):1377-85. doi: 10.1093/eurheartj/ehn153.
ouvrir dans Pubmed - Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018 Oct 4. pii: dci180033. doi: 10.2337/dci18-0033.
ouvrir dans Pubmed - Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018 Dec;41(12):2669-2701. doi: 10.2337/dci18-0033.
ouvrir dans Pubmed - Neumiller JJ, White JR Jr, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs 2010 Mar 5;70(4):377-85.
ouvrir dans Pubmed - Buysschaert M. La dapagliflozine (Forxiga®) : un nouvel inhibiteur des SGLT-2. Quelle position dans le traitement moderne du diabète de type 2 ? Louvain Med. 2016; 135 (9): 543-549.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28.
ouvrir dans Pubmed - Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. ; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 Aug 17;377(7):644-657.
ouvrir dans Pubmed - Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW et al.; CVD-REAL Investigators and Study Group. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose- Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017 Jul 18;136(3):249-259.
ouvrir dans Pubmed - Birkeland K, Jørgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucoselowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017 Sep;5(9):709-717.
ouvrir dans Pubmed - Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al.; for DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2018 Nov 10. doi: 10.1056/NEJMoa1812389.
ouvrir dans Pubmed - Buysschaert M. Les SGLT-2 inhibiteurs sont-ils une approche thérapeutique holistique du diabète de type 2 ? Le point après l’essai EMPA-REG OUTCOME. Louvain Med. 2017 ; 136 : 293-299.
- Buysschaert M. L’empagliflozine (Jardiance®), un nouvel hypoglycémiant dans le traitement du diabète de type 2, diminue aussi le risque cardiovasculaire : analyse d’une étude princeps. Louvain Med. 2015; 134 (8): 403-408.
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M ; for EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375: 323-334.
ouvrir dans Pubmed - Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2018 Nov 9. pii: S0140-6736(18)32590-X. doi: 10.1016/S0140-6736(18)32590-X
ouvrir dans Pubmed - Verma S, Jüni P, Mazer CD. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet. 2018 Nov 9. pii: S0140-6736(18)32824-1. doi: 10.1016/S0140-6736(18)32824-1.
ouvrir dans Pubmed